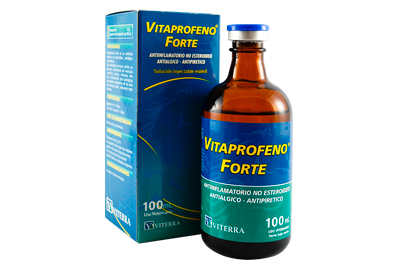
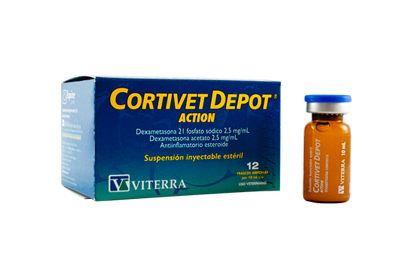
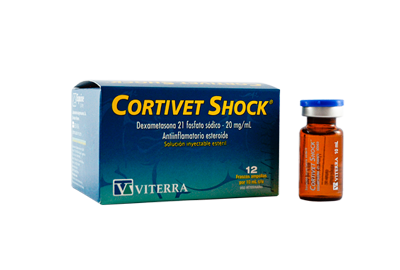
This treatment accomplishes:
- Eliminate acute and chronical inflamatory processes of different ethiology.
- High analgesic and antipyretic power.
- Easy administration IV and IM (Vitaprofeno)
- Excellent alternative in preventing or treating laminitis (Vitaprofeno)
- Eliminate acute and chronical inflamatory processes of different ethiology.
- High analgesic and antipyretic power.
- Easy administration IV and IM (Vitaprofeno)
- Excellent alternative in preventing or treating laminitis (Vitaprofeno)